Abstract
Background
Improvement of the efficacy and safety of chimeric antigen receptor (CAR)-T immunotherapies requires controlled activation and termination of the T cells when transfused into patients. Here we present two independently regulated molecular switches that can elicit specific and rapid induction of cellular responses upon exposure to their cognate ligands. T cell costimulation is controlled by the homodimerizer rimiducid that triggers signaling cascades downstream of MyD88 and CD40 via an engineered protein termed iMC. A rapamycin-controlled pro-apoptotic switch (iRC9) that induces dimerization of caspase-9 mitigates possible CAR-T cell toxicity. iRC9 is a chimeric protein comprisingan FKBP-rapamycin binding (FRB) domain in tandem with FKBP12 and caspase-9. This design permits rapamycin, a heterodimerizing ligand, to function as a homodimerizer. When combined with a first generation CD123-specific CAR, these molecular switches allow for controlled, robust expansion of engineered T cells to control acute myelogenous leukemia (AML) in vitro and in vivo combined with a rapid and efficient safety mechanism to block excessive cytokine release.
Methods & Results
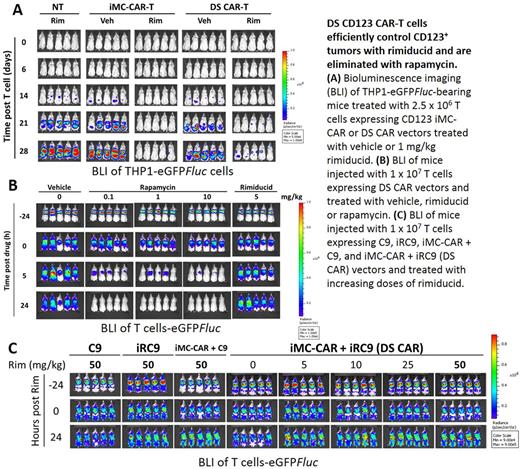
T cells were activated and co-transduced with pSFG-iMC.2A.CD123CAR.ζ and pSFG-iRC9.2A.ΔCD19 vectors to generate Dual-switch (DS) CAR-T cells. Combined transduction of iRC9 and iMC-CAR vectors produced CD123-directed CAR-T cells that eliminated CD123+ THP1 and MOLM13 AML cells, but not CD123- HPAC tumor cells, in a co-culture assay. Cytokine secretion and target cell killing were dependent on the dose of rimiducid (EC50 < 0.5 nM) to activate iMC costimulation. When challenged in a THP1-eGFP Fluc tumor-bearing mouse xenograft model, activation of the on-switch by rimiducid (1 mg/kg) in DS CAR-T cells enhanced tumor killing measured by bioluminescence imaging and T-cell expansion determined by splenocyte flow cytometry and vector copy number analyses.
Deployment of the off-switch induced fast (½ Vmax ~ 8 hours) and efficient T cell elimination of in a caspase-3 activation assay with real-time monitoring by Incucyte microscopy as well as Annexin V detection by flow cytometry (DS CAR-T = 77.6% versus untransduced = 2.2% Annexin V+ when treated with 1 nM rapamycin). In vivo assessment of the suicide switch was performed with eGFP Fluc -labeled CD123 DS CAR-T cells in NSG mice. Rapamycin, but not rimiducid, treatment efficiently eliminated DS CAR-T cells within 24 hours in NSG mice, which is similar to the clinically validated rimiducid-regulated iC9 switch. Importantly, the off-switch was insensitive to high rimiducid concentration, demonstrating that the on-switch regulator does not crosstalk with the safety switch.
Summary
Dual switch CAR-T, a novel platform comprising a first-generation CAR combined with regulated activation and apoptotic signaling elements, effectively controlled tumor growth and T cell expansion and elimination in vitro and in vivo . This dual switch technology provides a user-controlled system for managing persistence and safety of tumor antigen-specific CAR-T cells.
Bayle: Bellicum Pharmaceuticals: Employment, Equity Ownership. Duong: Bellicum Pharmaceuticals: Employment, Equity Ownership. Lu: Bellicum Pharmaceuticals: Employment. Morschl: Bellicum Pharmaceuticals: Employment, Equity Ownership. Collinson-Pautz: Bellicum Pharmaceuticals: Employment, Equity Ownership. Sharp: Bellicum Pharmaceuticals: Employment, Equity Ownership. Szymanski: Bellicum Pharmaceuticals: Employment, Equity Ownership. Brandt: Bellicum Pharmaceuticals: Employment, Equity Ownership. Slawin: Bellicum Pharmaceuticals: Consultancy, Equity Ownership. Toler: Bellicum Pharmaceuticals: Employment, Equity Ownership. Yvon: Bellicum Pharmaceuticals: Equity Ownership. Foster: Bellicum Pharmaceuticals: Employment, Other: stockholders . Spencer: Bellicum Pharmaceuticals: Employment, Equity Ownership, Other: stockholders .
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal